1. \[H_{2}O_{2}\] is commonly prepared in lab. by the reaction of
a) \[PbO_{2}+H_{2}SO_{4}\]
b) \[MnO_{2}+H_{2}SO_{4}\]
c) \[BaO_{2}+H_{2}O+CO_{2}\]
d) \[Na_{2}O_{2}+H_{2}O\]
Explanation: \[BaO_{2}+H_{2}O+CO_{2}\] \[\rightarrow\] \[BaCO_{3}+H_{2}O_{2}\] (Merck process)
2.Calculate the normality of 10 volume \[H_{2}O_{2}\]
a) 1.7 N
b) 12 N
c) 30.3 N
d) 0.0303 N
Explanation:
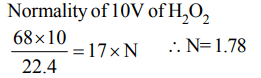
3. Commercial 10 volume \[H_{2}O_{2}\] is a solution with a strength of approximately
a) 30%
b) 3%
c) 1%
d) 10%
Explanation:
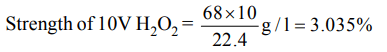
4. A 6 volume sample of \[H_{2}O_{2}\]
a) will contain 6% V/V of \[H_{2}O_{2}\]
b) will contain 6% W/V of \[H_{2}O_{2}\]
c) would give 6 volumes of oxygen per unit volume of \[H_{2}O_{2}\] sample at STP
d) would give 6 volumes of oxygen per unit weight of \[H_{2}O_{2}\] sample at STP
Explanation: 6 volume H2O2 would give 6 volumes of O2 per unit volume of H2O2
5. The structure of \[H_{2}O_{2}\] is
a) planar
b) non planar
c) spherical
d) linear
Explanation: Structure of H2O2 is nonplanar
6. In which of the following reactions, \[H_{2}O_{2}\] is acting as a reducing agent
a) \[H_{2}O_{2}+SO_{2}\rightarrow H_{2}SO_{4}\]
b) \[2KI+H_{2}O_{2}\rightarrow2KOH+ I_{2}\]
c) \[PbS+4H_{2}O_{2}\rightarrow PbSO_{4}+4 H_{2}O\]
d) \[Ag_{2}O+H_{2}O_{2}\rightarrow 2Ag+ H_{2}O+O_{2}\]
Explanation: SO2 changes to H2 SO4 (O.N. changes from +4 to +6 oxidation)
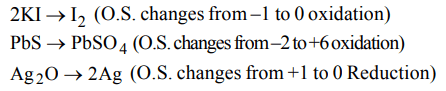
7. Which of the following is formed by the action of water on sodium peroxide
a) \[H_{2}\]
b) \[N_{2}\]
c) \[O_{2}\]
d) \[CO_{2}\]
Explanation:
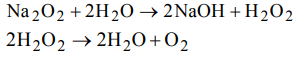
8. The percentage by weight of hydrogen in \[H_{2}O_{2}\] is
a) 5.88
b) 6.25
c) 25
d) 50
Explanation:
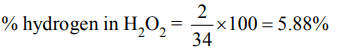
9. In which of the following reactions, \[H_{2}O_{2}\] acts as a reducing agent?
a) \[PbO_{2}\left(s\right)+H_{2}O_{2}\left(aq\right)\rightarrow PbO\left(s\right)+ H_{2}O\left(l\right)+O_{2}\left(g\right)\]
b) \[Na_{2}SO_{3}\left(aq\right)+H_{2}O_{2}\left(aq\right)\rightarrow Na_{2}SO_{4}\left(aq\right)+ H_{2}O\left(l\right)\]
c) \[2KI\left(aq\right)+H_{2}O_{2}\left(aq\right)\rightarrow 2KOH\left(aq\right)+ I_{2}\left(s\right)\]
d) \[KNO_{2}\left(aq\right)+H_{2}O_{2}\left(aq\right)\rightarrow KNO_{3}\left(aq\right)+ H_{2}O\left(l\right)\]
Explanation: PbO2 \[\rightarrow\] PbO (change in O.S. is +4 to +2 hence reduction)
10. The low density of ice compared to water is due to
a) hydrogen bonding interactions
b) dipole – dipole interactions
c) dipole – induced dipole interactions
d) induced dipole – induced dipole interactions
Explanation: It is due to hydrogen bonding when H2O forms a cage like structure in solid ice and density is reduced