1. The critical temperature of water is higher than that of \[O_{2}\] because \[H_{2}O\] molecule has
a) fewer electrons than oxygen
b) two covalent bonds
c) v-shape
d) dipole moment
Explanation: Critical temperature of water is more than O2 due to its dipole moment (Dipole moment of water = 1.84 D; Dipole moment of O2 = zero).
2. Hydrogen is not obtained when Zn reacts with
a) cold water
b) dil \[H_{2}SO_{4}\]
c) dil. HCl
d) 20% NaOH
Explanation: Only elements having reduction potential less than - 0.41V liberate hydrogen with cold water.
3. True peroxide is
a) \[BaO_{2}\]
b) \[MnO_{2}\]
c) \[PbO_{2}\]
d) \[NO_{2}\]
Explanation:
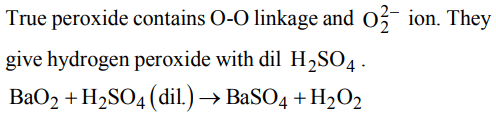
4. An inorganic compound gives off O2 when heated, turns an acidic solution of KI violet and reduces acidified \[KMnO_{2}\] . The compound is
a) \[SO_{3}\]
b) \[KNO_{3}\]
c) \[H_{2}O_{2}\]
d) All of these
Explanation:
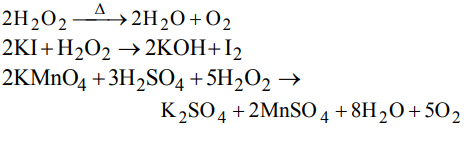
5. Acidified solution of chromic acid on treatment with \[H_{2}O_{2}\] yields
a) \[CrO_{3}+H_{2}O+O_{2}\]
b) \[Cr_{2}O_{2}+H_{2}O+O_{2}\]
c) \[CrO_{5}+H_{2}O+K_{2}SO_{4}\]
d) \[H_{2}Cr_{2}O_{7}+H_{2}O+O_{2}\]
Explanation:
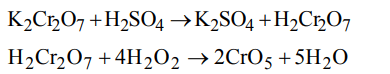
6. The m.pt. of most of the solid substances increase with an increase of pressure. However ice melts at a temperature lower than its usual melting point when pressure is increased. This is because
a) ice is less denser than \[H_{2}O\]
b) pressure generates heat
c) the chemical bonds break under pressure
d) ice is not a true solid
Explanation:
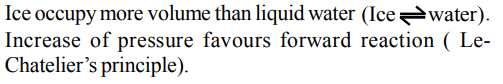
7. Acidified K2Cr2O7 on oxidation by \[H_{2}O_{2}\] gives
a) Blue solution
b) \[CrO_{5}\]
c) Chromium peroxide
d) All of these
Explanation:
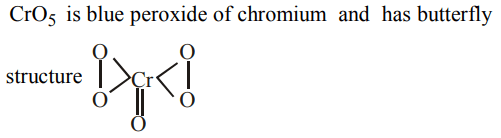
8.Which reaction shows oxidising nature of \[H_{2}O_{2}\]
a) \[H_{2}O_{2}+2KI\rightarrow 2KOH+I_{2}\]
b) \[Cl_{2}+H_{2}O_{2}\rightarrow2HCl+O_{2}\]
c) \[H_{2}O_{2}+Ag_{2}O\rightarrow2Ag+H_{2}O+O_{2}\]
d) \[NaClO+H_{2}O_{2}\rightarrow NaCl+H_{2}O+O_{2}\]
Explanation:
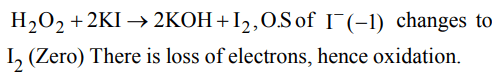
9. The volume strength of 0.5 N \[H_{2}O_{2}\] solution is
a) 4.8
b) 8.4
c) 3.0
d) 8.0
Explanation:
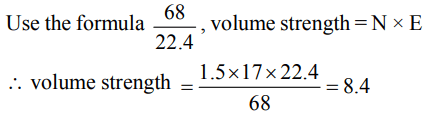
10. Calgon is an industrial name given to
a) normal sodium phosphate
b) sodium meta aluminate
c) sodium hexametaphosphate
d) hydrated sodium aluminium silicate
Explanation: Sodium hexametaphosphate (Na6P6O18) is calgon, it is used to remove permanent hardness of water.