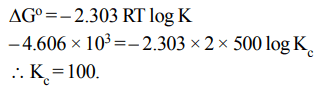1. When 3 mole of reactant A and one mole of the reactant B are mixed in a vessel of volume 1 litre, the following reaction takes place
\[A\left(g\right)+B\left(g\right)\rightleftharpoons 2C\left(g\right)\] If 1.5 mole of C is formed at equilibrium, the equilibrium constant \[\left(K_{C}\right)\] of the reaction is
a) 0.12
b) 0.50
c) 0.25
d) 4.00
Explanation:
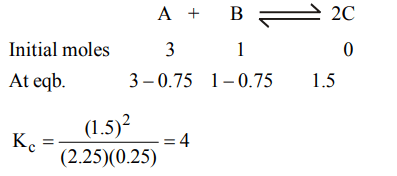
2. Kc for the reaction \[N_{2}\left(g\right)+O_{2}\left(g\right)\rightleftharpoons 2NO\left(g\right)\] at 300 K is \[4.0 × 10^{-6}\] . \[K_{P}\] for the above reaction will be \[\left(R=2 cal mol^{-1}K^{-1}\right)\]
a) \[2.4 × 10^{-3}\]
b) \[4 × 10^{-6}\]
c) \[4 × 10^{-6}\left(RT\right)^{2}\]
d) \[16 × 10^{-12}\]
Explanation:
3. Which of the following equilibria will shift to right side on increasing the temperature?
a) \[CO \left(g\right)+H_{2}O\left(g\right)\rightleftharpoons CO_{2} \left(g\right)+H_{2}\left(g\right) \]
b) \[2SO_{2} \left(g\right)+O_{2}\left(g\right)\rightleftharpoons 2SO_{3}\left(g\right) \]
c) \[H_{2}O\left(g\right)\rightleftharpoons H_{2}\left(g\right)+1\diagup2 O_{2} \left(g\right)\]
d) \[4HCI\left(g\right)+O_{2}\left(g\right)\rightleftharpoons {2}H_{2}O\left(g\right)+2CI_{2} \left(g\right)\]
Explanation: Reaction (c) is endothermic. Electrolysis or decomposition of H2O is endothermic in nature.
4. 1 mole of \[N_{2}\] and 2 moles of \[H_{2}\] are allowed to react in a \[1 dm^{3}\] vessel. At equilibrium 0.8 mole of \[NH_{3}\] is formed. The
concentration of \[H_{2}\] in the vessel is
a) 0.6 mole
b) 0.8 mole
c) 0.2 mole
d) 0.4 mole
Explanation:
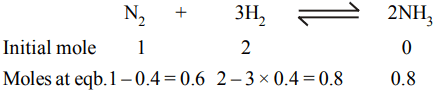
5. The equilibrium \[SO_{2}Cl_{2}\left(g\right)\rightleftharpoons SO_{2}\left(g\right) + Cl_{2}\left(g\right)\] is attained at 25°C in a closed container and an inert gas,
helium is introduced. Which of the following statement is correct
a) More chlorine is formed
b) Concentration of \[SO_{2}\] is reduced
c) More \[SO_{2}Cl_{2}\] is formed
d) Concentration of \[SO_{2}Cl_{2},SO_{2} and Cl_{2}\] do not change
Explanation: No change in concentration at constant volume only pressure is increased when some inert gas is introduced.
6. For which reaction high pressure and high temperature is helpful in obtaining a high equilibrium yield
a) \[2NF_{3}\left(g\right)\rightleftharpoons N_{2}\left(g\right)+3F_{2}\left(g\right) – 54.40 kcal\]
b) \[N_{2}\left(g\right)+3H_{2}\left(g\right) \rightleftharpoons 2NH_{3}\left(g\right) + 22.08 kcal\]
c) \[Cl_{2}\left(g\right)+2O_{2}\left(g\right) \rightleftharpoons 2ClO_{2}\left(g\right) – 49.40 kcal\]
d) \[2Cl_{2}O_{7}\left(g\right) \rightleftharpoons 2Cl_{2}\left(g\right) +7O_{2}\left(g\right)+ 126.8 kcal\]
Explanation: \[Cl_{2}\left(g\right)+2O_{2}\left(g\right) \rightleftharpoons 2ClO_{2}\left(g\right) – 49.40 kcal\]
7. For the reversible reaction \[N_{2}\left(g\right)+3H_{2}\left(g\right) \rightleftharpoons2NH_{3}\left(g\right)\] at 500°C, the value of \[K_{P}\] is \[1.44 × 10^{-5}\] when partial pressure
is measured in atmospheres. The corresponding value of \[K_{C}\] with concentration in mole litre-1, is
a) \[1.44 × 10^{-5}/ (0.082 × 500)^{-2}\]
b) \[1.44 × 10^{-5}/ (8.314 × 773)^{-2}\]
c) \[1.44 × 10^{-5}/ (0.082 × 773)^{2}\]
d) \[1.44 × 10^{-5}/ (0.082 × 773)^{-2}\]
Explanation:
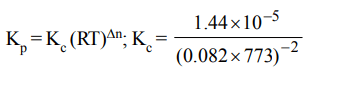
8. A 1 M solution of glucose reaches dissociation equilibrium according to the equation given below \[6HCHO\rightleftharpoons C_{6}H_{12}O_{6}\] .
What is the concentration of HCHO at equilibrium if equilibrium constant is \[6 × 10^{22}\]
a) \[1.6 × 10^{-8}M\]
b) \[3.2 × 10^{-6}M\]
c) \[3.2 × 10^{-4}M\]
d) \[1.6 × 10^{-4}M\]
Explanation:
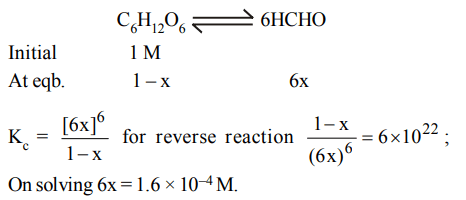
9. 8 mole of a gas \[AB_{3}\] are introduced into a 1.0 dm3 vessel. It dissociates as,\[2AB_{3}\left(g\right)\rightleftharpoons A_{2}\left(g\right) + 3B_{2}\left(g\right)\] . At
equilibrium, 2 mole of \[A_{2}\] are found to be present. The equilibrium constant of reaction is ........ in \[mol^{2}L^{-2}\]
a) 2
b) 3
c) 27
d) 36
Explanation:
10. \[\triangle G^{\circ}\] for the reaction \[X + Y\rightleftharpoons Z\] is –ve 4.606 kcal. The equilibrium constant for the reaction at 227°C is.
a) 100
b) 10
c) 2
d) 0.01
Explanation: