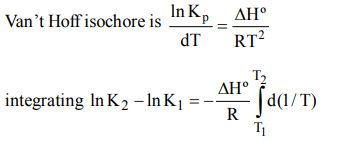1. In a chemical reaction calculate rate constant of backward reaction when the rate constant of forward reaction is 20 and the equilibrium constant is 50
a) (0.2)
b) (0.1)
c) (0.4)
d) None of these
Explanation:
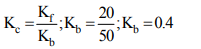
2. The rate constant is given by the equation \[K=PZe^{-E/RT}\] . Which factor should register a decrease for the reaction to proceed more rapidly?
a) T
b) Z
c) E
d) P
Explanation: The lower the energy of activation, the more is the rate of reaction
3. For the reaction \[CO\left(g\right)+H_{2}O\left(g\right)\rightleftharpoons CO_{2}\left(g\right)+H_{2}\left(g\right)\] at a given temperature the equilibrium amount of \[CO_{2}\left(g\right)\] can be
increased by
a) adding a suitable catalyst
b) adding an inert gas
c) decreasing the volume of the container
d) increasing the amount of \[CO\left(g\right)\]
Explanation: increasing the amount of \[CO\left(g\right)\]
4. At constant temperature, the equilibrium constant \[\left(K_{P}\right)\] for the decomposition reaction \[N_{2}O_{4}\rightleftharpoons2NO_{2}\] is expressed
by \[K_{P}=\left(4x^{2}P\right)/\left(1-x^{2}\right)\] , where P = pressure, x = extent of decomposition. Which one of the following statements is true?
a) \[K_{P}\] increases with increase of P
b) \[K_{P}\] increases with increase of x
c) \[K_{P}\] increases with decrease of x
d) \[K_{P}\] remains constant with change in P and x
Explanation: With change of pressure, x will change in such a way that Kp remains constant
5. Consider the following equilibrium in a closed container \[N_{2}O_{4}\left(g\right)\rightleftharpoons2NO_{2}\left(g\right)\] At a fixed temperature, the volume of the reaction container is halved. For this change, which of the following statements holds true regarding the equilibrium
constant \[\left(K_{P}\right)\] and degree of dissociation \[\left(\alpha\right)\]
a) \[K_{P}\] does not changes, but \[\alpha\] changes
b) \[K_{P}\] changes, but \[\alpha\] does not change
c) both \[K_{P}\] and \[\alpha\] change
d) neither \[K_{P}\] nor \[\alpha\] changes
Explanation: For the equilibria: \[N_{2}O_{4}\left(g\right)\rightleftharpoons2NO_{2}\left(g\right)\]
\[K_{P} = K_{C} \times (RT)^{\triangle n}\] Since temperature is constant so KC or KP will remain constant. Further since volume is halved, the pressure will be doubled so a will decrease so as to maintain the constancy of KC or KP .
6. Which of the following is true at chemical equilibrium?
a) \[\left(\triangle G\right)_{T,P}\] is minimum and \[\left(\triangle S\right)_{U,V}\] is also minimum
b) \[\left(\triangle G\right)_{T,V}\] is minimum and \[\left(\triangle S\right)_{U,V}\] is maximum
c) \[\left(\triangle G\right)_{T,V}\] is maximum and \[\left(\triangle S\right)_{U,V}\] is zero
d) \[\left(\triangle G\right)_{T,P}\] is zero and \[\left(\triangle S\right)_{U,V}\] is also zero
Explanation: The condition for the equilibrium is
\[({\triangle G})_{T,P}\] = 0 and \[({\triangle S})_{U,V}\] = 0
7. For the reaction \[A\left(g\right)\rightleftharpoons B\left(g\right)+C\left(g\right)\],
a) \[K_{P}=\alpha^{3}P\]
b) \[K_{P}=\alpha^{2}\left(K_{P}+P+1\right)\]
c) \[K_{P}=\alpha^{2}\left(K_{P}+P\right)\]
d) \[K_{P}=\alpha^{2}\left[\frac{K_{P}+P}{P}\right]\]
Explanation:
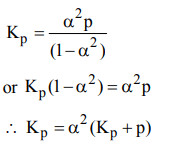
8. In the van’t Hoff equation
\[\frac{dlnK}{dT}=\frac{\triangle H^{\circ}}{RT^{2}}\]
a) when \[\frac{dlnK}{dT}<0\] , the reaction is exothermic
b) when dlnK \[\frac{dlnK}{dT}<0\] , the reaction is endothermic
c) the slope of the graph is positive throughout
d) the slope of graph increases and then decreases
Explanation:
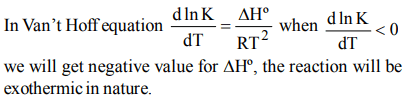
9. Consider the expression \[\triangle G=–RTlnK_{P}+RTlnQ_{P}\] and indicate the correct statement at equilibrium
a) \[\triangle G=0,Q_{P}>K_{P}\] the equilibrium reaction will shift from left to right
b) \[\triangle G=0,Q_{P}=K_{P}\] the equilibrium reaction will shift from left to right
c) \[\triangle G=\propto,Q_{P}<K_{P}\] the equilibrium reaction will shift from right to left
d) \[\triangle G<0,Q_{P}>K_{P}\] the equilibrium reaction will shift from right to left
Explanation:

10. By which of the following reactions, the equilibrium constant
is related to tempearture?
a) \[ln K_{2}-ln K_{1}=\frac{\triangle H^{\circ}}{R}\int_{T_{1}}^{T_{2}} d\left(\frac{1}{T}\right)\]
b) \[ln K_{2}-ln K_{1}=-\frac{\triangle H^{\circ}}{R}\int_{1/T_{1}}^{1/T_{2}} d\left(\frac{1}{T^{2}}\right)\]
c) \[ln K_{2}-ln K_{1}=-\frac{\triangle H^{\circ}}{R}\int_{T_{1}}^{T_{2}} d\left(\frac{1}{T}\right)\]
d) \[ln K_{2}-ln K_{1}=-\frac{\triangle H^{\circ}}{R}\int_{1/T_{2}}^{1/T_{1}} d\left(\frac{1}{T}\right)\]
Explanation: