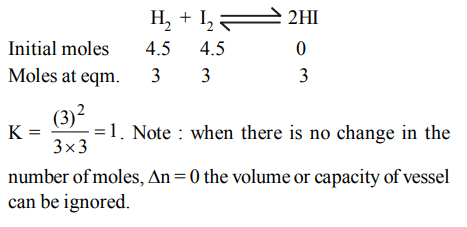1. In the equilibrium reaction involving the dissociation of \[CaCO_{3}\]
\[CaCO_{3}\left(s\right)\rightleftharpoons CaO\left(s\right)+CO_{2}\left(g\right)\]
the equilibrium constant is given by
a) \[\frac{p_{CaO}*p_{CO_{2}}}{p_{CaCO_{3}}}\]
b) \[C_{CaO}*\frac{p_{CO_{2}}}{C_{CaCO_{3}}}\]
c) \[\frac{p_{CaO}}{p_{CaCO_{3}}}\]
d) \[p_{CO_{2}}\]
Explanation: Kp = \[p_{CO_{2}}\] others are solids. The concentration terms for solids and liquids are taken as unity
2. Steam reacts with iron at high temperature to give hydrogen gas and \[Fe_{3}O_{4}\left(s\right)\] . The correct expression for the equilibrium constant is
a) \[\frac{P_{H_{2}}^2}{P_{H_{2}O}^2}\]
b) \[\frac{\left(P_{H_{2}}\right)^{4}}{\left(P_{H_{2}O}\right)^{4}}\]
c) \[\frac{\left(P_{H_{2}}\right)^{4}\left[Fe_{3}O_{4}\right]}{\left(P_{H_{2}O}\right)^{4}\left[Fe\right]}\]
d) \[\frac{\left[Fe_{3}O_{4}\right]}{\left[Fe\right]}\]
Explanation:
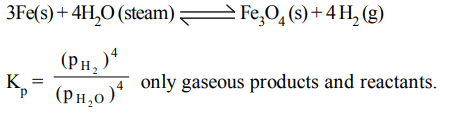
3. In lime kiln, the reversible reaction
\[CaCO_{3}\left(s\right)\rightleftharpoons CaO\left(s\right)+CO_{2}\left(g\right)\]
proceeds to completion because
a) of high temperature
b) \[CO_{2}\] escaped out
c) CaO is removed
d) of low temperature
Explanation: Forward reaction is favoured by removal of products
4. If 1.0 mole of \[I_{2}\] is introduced into 1.0 litre flask at 1000 K, at quilibrium \[\left(K_{C}=10^{-6}\right)\] , which one is correct
a) \[\left[I_{2}\left(g\right)\right]>\left[I^{-}\left(g\right)\right]\]
b) \[\left[I_{2}\left(g\right)\right]<\left[I^{-}\left(g\right)\right]\]
c) \[\left[I_{2}\left(g\right)\right]=\left[I^{-}\left(g\right)\right]\]
d) \[\left[I_{2}\left(g\right)\right]=\frac{1}{2}\left[I^{-}\left(g\right)\right]\]
Explanation:
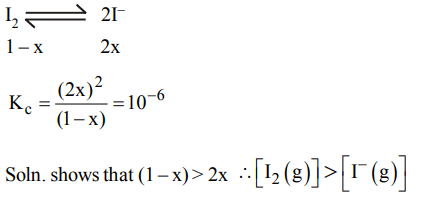
5. At equilibrium, if\[K_{P}=1\] , then
a) \[\triangle G^{\circ}=0\]
b) \[\triangle G^{\circ}>1\]
c) \[\triangle G^{\circ}<1\]
d) None of these
Explanation:
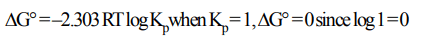
6. Van't Hoff's equation giving the effect of temperature on chemical equilibrium is represented as
a) \[\frac{d In F}{dT}=\frac{\triangle H}{RT^{2}}\]
b) \[\frac{d ln K_{P}}{dT}=\frac{\triangle HT^{2}}{R}\]
c) \[\frac{d ln K_{P}}{dT}=\frac{\triangle H}{RT^{2}}\]
d) \[\frac{d ln K_{P}}{dT}=\frac{RT^{2}}{\triangle H}\]
Explanation:
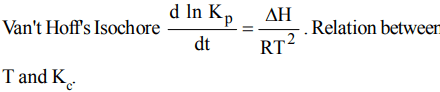
7. Solubility of a substance which dissolves with a decrease in volume and absorption of heat will be favoured by
a) high P and high T
b) low P and low T
c) high P and low T
d) low P and high T
Explanation: high P and high T
8. In what manner will increase of pressure affect the following equation?
\[C\left(s\right)+H_{2}O\left(g\right)\rightleftharpoons CO\left(g\right)+H_{2}\left(g\right)\]
a) Shift in the forward direction
b) Shift in the reverse direction
c) Increase in the yield of hydrogen
d) No effect
Explanation: Reverse reaction, Le Chatelier's principle \[\triangle\]n=2 – 1 = 1.
9. In a reaction, \[A + 2B\rightleftharpoons 2C\] , 2.0 mole of 'A', 3.0 mole of 'B' and 2.0 mole of 'C' are placed in a 2.0 L flask and the equilibrium concentration of 'C' is 0.5 mole/L. The equilibrium constant (K) for the reaction is
a) 0.073
b) 0.147
c) 0.05
d) 0.026
Explanation:
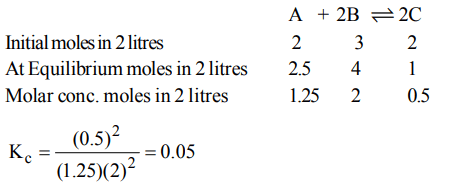
10. 4.5 moles each of hydrogen and iodine were heated in a sealed ten litre vessel. At equilibrium 3 moles of HI were found. The equilibrium constant of
\[H_{2}\left(g\right)+I_{2}\left(g\right)\rightleftharpoons 2HI\left(g\right)\]
a) 1
b) 10
c) 3
d) 0.33
Explanation: