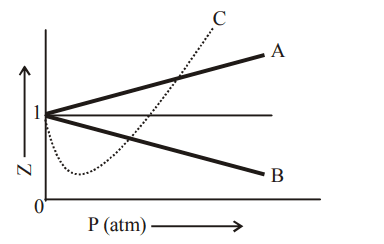
a) For the gas A, a = 0 and its dependence on P is linear at all pressure
b) For the gas B, b = 0 and its dependence on P is linear at all pressure
c) For the gas C, which is typical real gas for which neither a nor b = 0. By knowing the minima and the point of intersection, with Z = 1, a and b can be calculated
d) At high pressure, the slope is positive for all real gases
Answer: b
Explanation: For the gas B, b = 0 and its dependence on P is linear at all pressure
Join The Discussion