a) \[0.253L^{2}mol^{-2}atm\]
b) \[0.53L^{2}mol^{-2}atm\]
c) \[1.853L^{2}mol^{-2}atm\]
d) \[1.253L^{2}mol^{-2}atm\]
Answer: d
Explanation:
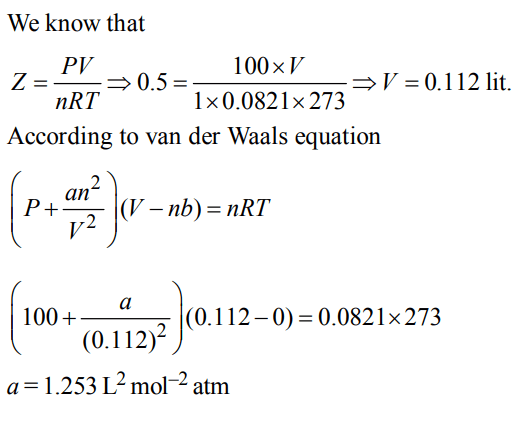
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Join The Discussion