a) \[\left(P_{A}\diagup P_{B}\right)\left(M_{B}\diagup M_{A}\right)^{1\diagup 2}\]
b) \[\left(P_{A}\diagup P_{B}\right)^{1\diagup 2} \left(M_{A}\diagup M_{B}\right)\]
c) \[\left(P_{A}\diagup P_{B}\right) \left(M_{A}\diagup M_{B}\right)^{1\diagup 2}\]
d) \[\left(P_{A}\diagup P_{B}\right)^{1\diagup 2} \left(M_{B}\diagup M_{A}\right)\]
Answer: a
Explanation:
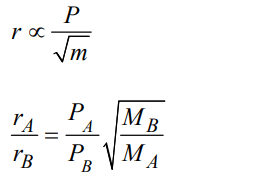
Join The Discussion