1. In a gas mixture if molecular weights of all the species are equal than which one will be more, Mass average velocity or Molar average velocity?
a) Mass average velocity
b) Molar average velocity
c) Both will have the same value
d) Nothing definite can be said
Explanation: Average molar mass of the mixture=M(x1+x2+………+ xn)=M
(x1+x2+………+ xn=1)
Mass average velocity=Σuiρi/ρ=uiPui/P
Molar average velocity=Σciui/c=ρuiPi/P (P=cRT)
2. Which among the following can be the unit of Flux?
a) Kg*m2*s-1
b) Kg*m-2*s-1
c) Kg*m*s2
d) Kg*m2*s1
Explanation: Dimension analysis
Flux= rate at which a species passes through a unit area, in unit time
3. Under which of the following conditions mass average velocity and molar average velocity are equal?
a) Always different
b) In a very dilute solution
c) If molecular weights of all the species are equal
d) In dilute solutions as well as in solutions in which all components have same molecular weights
Explanation: In dilute solutions as well as in solutions in which all components have same molecular weights
4. A solution contains 0.3 moles of solute A, 0.2 moles of B and 0.5 moles of C. What will be the mole fraction of A in the mixture?
a) 0.2
b) 0.3
c) 0.5
d) 1
Explanation: Mole Fraction of A= Moles of A/Total moles
5. Given diagram shows diffusion of water vapor through air. Identify the correct statement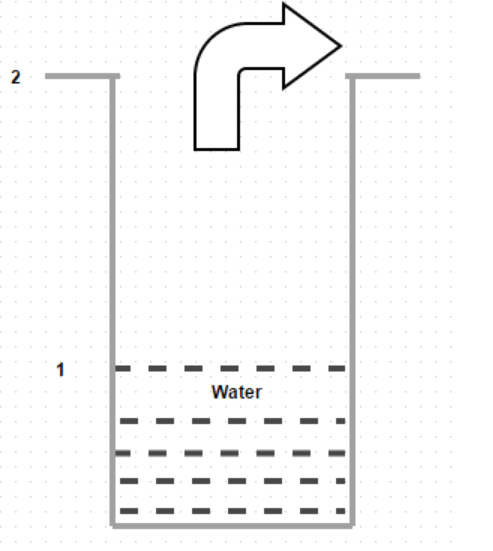
a) The water does not evaporates but diffuses upward
b) The water evaporates and diffuses downward
c) The water evaporates and diffuses upward
d) The water does not evaporates but diffuses downward
Explanation: The slight movement of air over the top of the tube does not bring about any change in the concentration profile of air.
6. In which of the following conditions mass transfer will occur spontaneously? C and z is concentration and distance respectively.
a) dC/dz<0
b) dC/dz>0
c) dC/dz=0
d) None of the Above
Explanation: Mass transfer occurs in the direction of decreasing concentration.
7. The velocity of various components of mixture(21% A, 78% B and 1% C) are:
Velocity is in the form: v=xi+yj+zk
Velocity of component A= (0.1, 0, 0)
Velocity of component B= (0, 0.1, 0)
Velocity of component B= (0, 0, 0.1)
Calculate the value of average molar velocity?
a) (0.079, 0.021, 0.01)
b) (0.021, 0.078, 0.01)
c) (0.1, 0.1, 0.1)
d) (0, 0, 0)
Explanation: Molar average velocity = (c1u1+c2u2+c3u3)/C
8. A gas mixture containing 21% O2 and 79% N2 flows through a pipe of circular cross-section of diameter 2cm at atmospheric pressure. The velocity of O2 is 0.04m/s and N2 is 0.035m/s. What is the value of molar average velocity (m/s) of the mixture?
a) 0.036
b) 0.033
c) 0.035
d) 0.034
Explanation: Molar average velocity= (C1u1+C2u2+……….)/C
=y1u1+y2u2…..
= 0.21*0.04+0.79*0.035
=0.036
9. For the calculation of Mass average velocity, which velocity of the molecule is used?
a) Bulk velocity of the mixture
b) Instantaneous Velocity
c) Mean Velocity
d) Instantaneous Velocity as well as Mean Velocity
Explanation: Mean Velocity
10. A gas mixture containing 21% O2 and 79% N2 flows through a pipe of circular cross-section of diameter 2cm at atmospheric pressure. The velocity of O2 is 0.04m/s and N2 is 0.035m/s. What will be the value of mass average velocity (m/s)?
a) 0.891
b) 0.981
c) 0.985
d) 0.857
Explanation: Mass average velocity= (ρ1u1+ρ2u2)/ ρ
ρ1= (p1*M1)/RT
ρ= (p*M)/RT.
Dividing these two equations
ρ1/ ρ = (p1*M1)/pM =(y1*M1)/M
M=y1*M1+y2*M2=0.79*28+0.21*32=28.84
Mass average velocity= (y1*M1*u1+y2*M2*u2)/M
= (0.21*32*0.04+0.79*0.035)/28.84
= 0.981