1. The temperature where the first vapor is formed, when heating a liquid consisting of two or more components
a) Dew point
b) Bubble point
c) Final point
d) Aniline point
Explanation: The temperature where the first vapor is formed when heating a liquid consisting of two or more components Bubble point, while when the first drop of aniline separates out is called the aniline point.
Nc∑Yi ∑Kixi.
2. The below equation holds for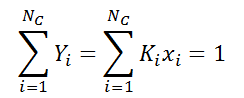
a) K and X relation
b) K and Bubble relation
c) Components and K values relation
d) Nc Relation
Explanation: At the bubble point, the following relationship holds always, representing Nc as number of components and yi as the mole fraction of all components.
3. The K in the below equation holds for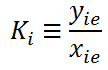
a) Distribution Factor
b) Henry Factor
c) Vapor Factor
d) Dew factor
Explanation: K is the distribution coefficient, defined as the ratio of mole fraction in the vapor phase, these are not always equal but are somehow equivalent to each other.
4. When Raoult’s law and Dalton’s law hold for the mixture, the K factor is
a) Pi/P
b) Yi/Xi
c) Y*/X
d) X*/X
Explanation: When Raoult’s law and Dalton’s law hold for the mixture, the K factor is Pi/P, indicating Pi as the partial pressure of system and P as total pressure.
5. The bubble point method is the most widely used for
a) Pore size determination
b) Bubble size determination
c) Stages determination
d) Tray determination
Explanation: The bubble point method is the most widely used for Pore size determination, Underwood, Gilliland, McCabe are used for stages and tray determination.
6. The BP Method generally work best for
a) Narrow Boiling systems
b) Ideal systems
c) Nearly ideal systems
d) Any systems
Explanation: The BP Method generally work for Narrow Boiling systems, Ideal systems and nearly ideal systems as boiling point of a component does not vary much as compared to other properties.
7. To perform a Bubble Point Test, gas is applied to
a) All the filter
b) Both side of wetted filter
c) One side of a wetted filter
d) Not specified
Explanation: To perform a Bubble Point Test, gas is applied to one side of a wetted filter, with the tubing downstream.
8. The equation below gives us the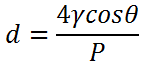
a) Reflux equation
b) Dew point equation
c) Stage efficiency equation
d) Capillary Rise equation
Explanation: The equation above gives us the Capillary Rise equation, d is the diameter of the capillary and P is the pressure applied and cos is the angle of inclination.
9. Bubble point equation is used for
a) Reflux and feed rate
b) Vapor and liquid composition
c) Venting and filtration
d) Equilibrium phases
Explanation: Bubble point equation is used for Venting and filtration purposes as the liquid evaporates the filtration of pure liquid can be achieved using condensation technique.
10. θ (theta) stands for
a) Convergence promoter
b) Catalyst promoter
c) Dynamic value
d) Bubble Extract point
Explanation: θ (theta) stands for Convergence promoter, TBp is defined as the bubble extract point and lambda as the catalyst parameter.